Wybierz swoją lokalizację i język, aby korzystać z najlepiej przystosowanej strony internetowej
Wybierz swoją lokalizację i język, aby korzystać z najlepiej przystosowanej strony internetowej

Compliance labeling is critical for manufacturers and distributors in many industries. From UDI or MDR labeling for medical devices to GHS labeling for chemicals to nutrition labeling for food and beverage products, there are a wide variety of labeling requirements. In emerging industries such as cannabis, labeling regulations vary by country or state and continue to develop.
Enforcement of compliance labeling varies too. Whether a government agency like the United States Food & Drug Administration (US FDA), Occupational Safety and Health Administration (OSHA), or the European Commission (EC) threatens fines for noncompliance, or your customers downstream in the supply chain will return improperly labeled shipments, you don’t want to be caught with noncompliant labels.
Managing compliance labeling doesn’t need to cause headaches when you use a robust and reliable enterprise labeling system. Following these key principles will set you up for compliance labeling success.
Whether you have 10 product varieties or 10,000, it’s inefficient to create a new label for each variety. However, this is what many companies end up doing when they don’t consider the scalability of their labeling system. When a new product variant or configuration is introduced, a new label file is created. Before long, you’re managing to hundreds or thousands of label files, and they’re often saved in random locations and updated inconsistently.
Using smart label templates connected to your business database eliminates this inefficiency and allows you to easily manage and update all your labels. Instead of thousands of individual label files, you can manage to a small number of templates that use database connections to pull in variable data such as lot number, SKU, shipping address, HazCom statements, health warnings, and even images. Simply update the data in the business database, and all labels using that data will be automatically updated.
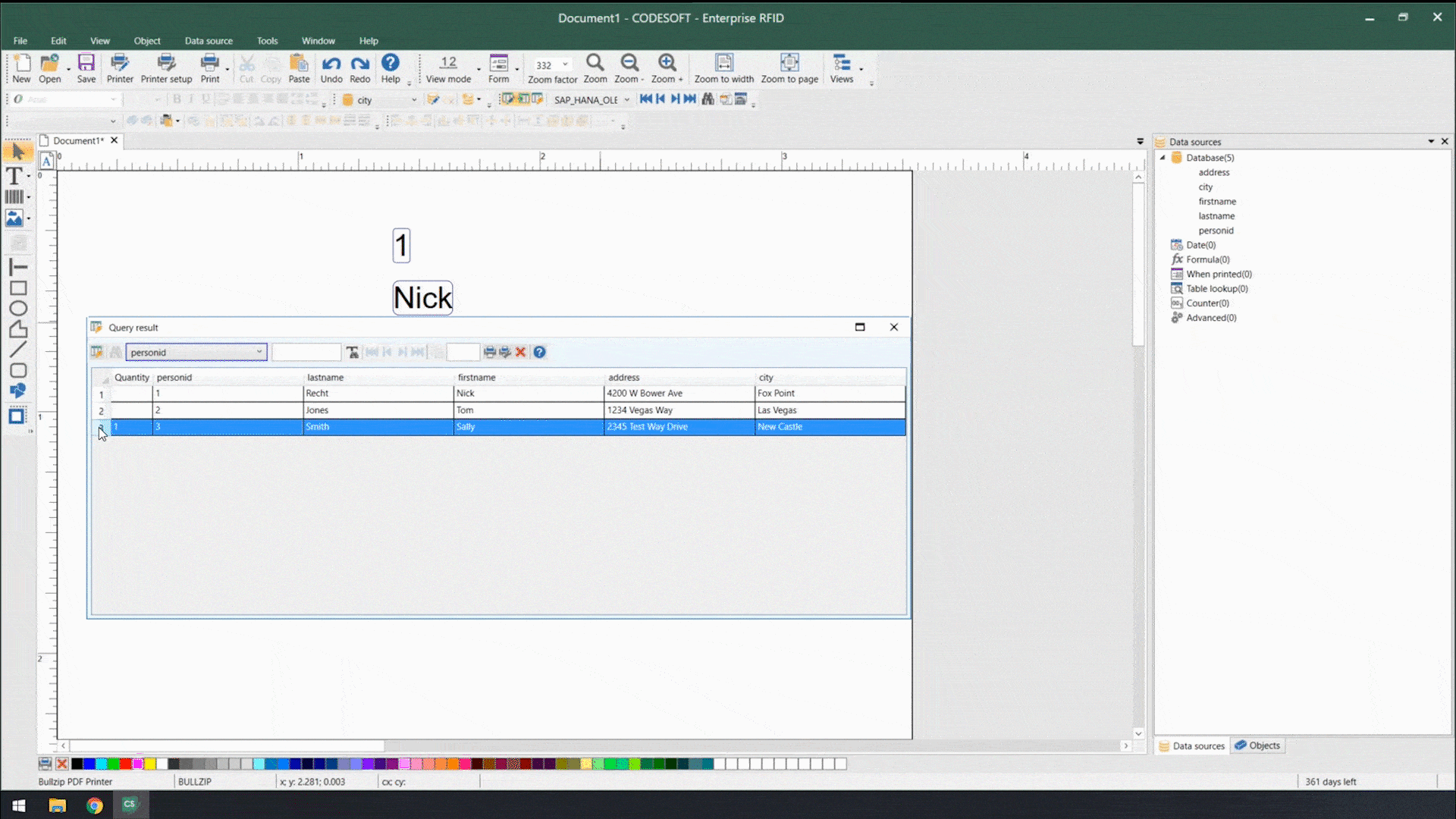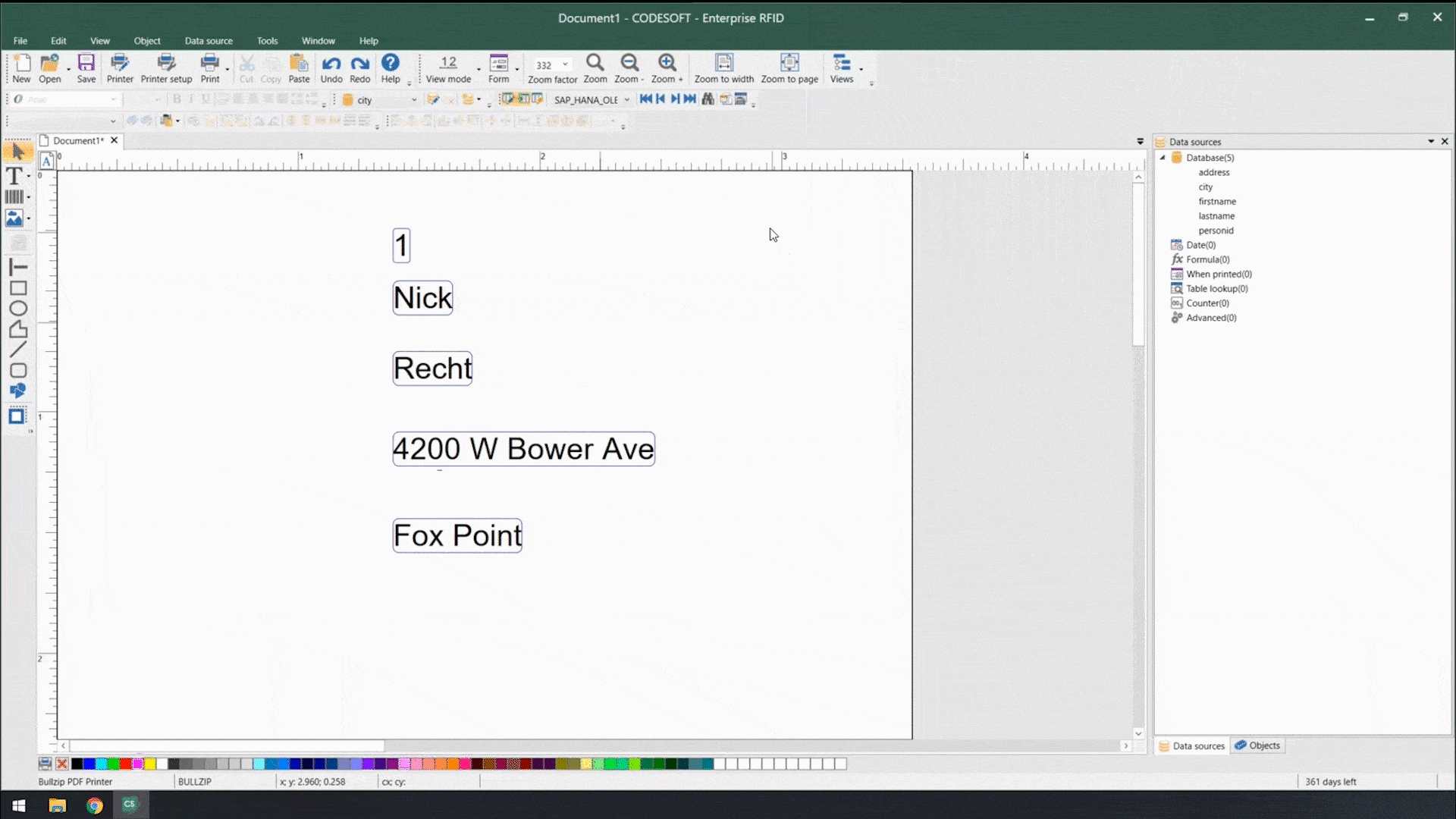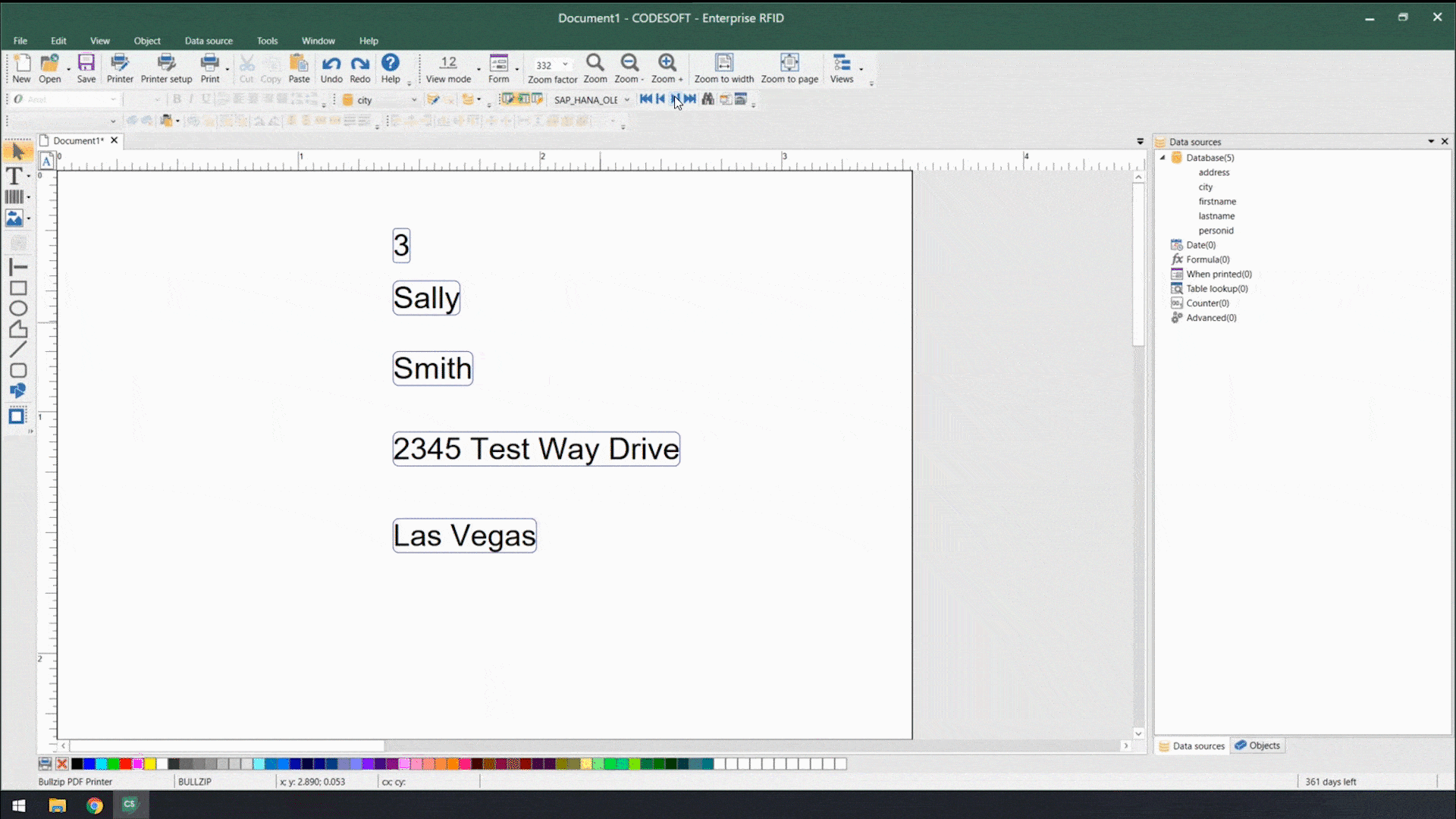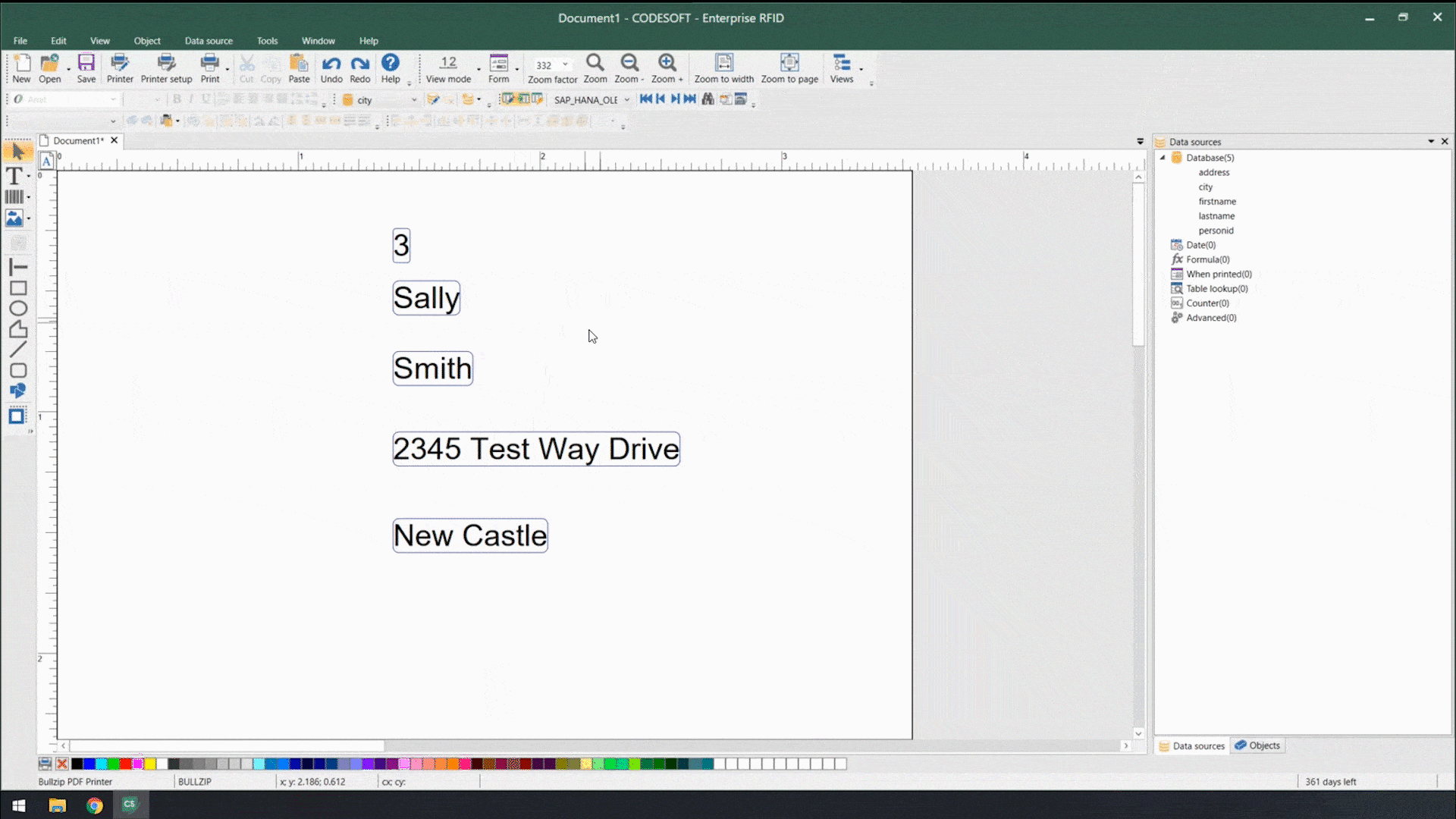
Smart label templates come in handy when labeling regulations change. Instead of having to open, edit, and save hundreds of files, you can update a handful of templates and be ready for updated regulations in no time.
If you are already maintaining hundreds or thousands of label files, don’t worry – TEKLYNX is here to help. We’ve helped countless companies consolidate their label files into templates, and we can help you too. Learn more.
Leveraging the power of smart label templates is the first step. An important next step is to ensure that unauthorized users can’t make changes and unapproved labels can’t be printed.
In an electronic label approval process, users are assigned specific permissions based on their role in labeling. A common set of label approval roles is:
Label designer – typically an employee who works in product management or in a regulatory compliance role
Label approver – typically one or more employees in leadership roles who are familiar with compliance labeling requirements, often in the quality assurance department
Label printer – typically a production floor worker who is printing and applying the final label
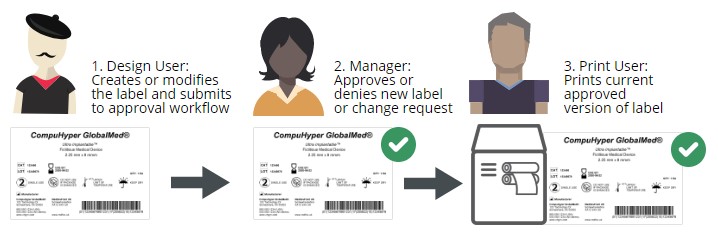
With clear roles and permissions assigned, labels can be quickly updated, approved, and printed with little risk of noncompliance. An electronic label approval process is much more efficient than printing labels and walking them around to each person in the process.
Some label regulations, such as FDA 21 CFR Part 11, require documentation of all label changes and approvals. Electronic signatures are required to prove that changes are accurate and compliant. With an electronic label approval process, the label approver can be prompted to enter a password, which is sufficient to meet the requirement of an electronic signature. All label changes, approvals, and print jobs are recorded in the system, making your company audit-ready.
In highly-regulated industries, regulatory authorities like the FDA and the European Union (EU) have guidelines related to process validation to confirm a company’s processes will result in reliable outputs and meet all necessary quality standards. The purpose of validation for labeling systems is to establish documented evidence that the labeling software is correctly installed, operates according to requirements, and performs safely. It also demonstrates that the manufacturing process, under normal operating conditions, will consistently produce conforming products and outputs.
Manufacturers in highly-regulated industries are often required to face (and perform) validation every three years. There are also a variety of events that may occur externally or internally that require labeling system validation documentation to be updated and/or inspected. TEKLYNX customers in these highly-regulated industries leverage LABEL ARCHIVE label traceability software and the TEKLYNX Validation Accelerator Pack to simplify and speed up labeling system validation.
Barcode quality is an important aspect of compliance labeling. Unfortunately, when it comes to grading and verifying barcodes, many companies use a spot-check method. They assume that if there’s an issue, it will show up on many labels and be caught by checking a few labels per print job. This haphazard process leaves those companies open to risk if a low-quality barcode slips through the cracks.
Luckily, there’s a better way. Did you know you can grade every single barcode that’s printed and track that information back into your enterprise labeling software? Enabled by a powerful partnership between TEKLYNX and TSC Printronix Auto ID, a complete end-to-end barcode verification system sets you up for compliance labeling success. Every single barcode is verified and graded, leaving no room for error on your barcode labels. Issues are promptly identified and flagged for resolution.
With this end-to-end barcode verification system, you’re equipped to create reports showing the verification and grades for every barcode you print.
Keeping labeling compliant with various government, industry, and supply chain requirements can be complex. You need a label system that equips you with the tools to make compliance labeling accurate, efficient, and scalable.
TEKLYNX offers LABEL ARCHIVE label security and traceability software. LABEL ARCHIVE leverages CODESOFT for efficient label design using smart label templates. It builds on CODESOFT’s robust label design capabilities by enforcing user roles and permissions for a completely electronic label approval process. LABEL ARCHIVE also comes equipped to receive and store data from TSC Printronix barcode verifiers, enabling end-to-end barcode verification.
Request a free consultation today to learn how LABEL ARCHIVE can help you manage compliance labeling for your company.
Travis Wayne is the Product Manager at TEKLYNX. Travis applies his 20+ years of IT and health sciences experience to empower businesses to streamline operations and barcode better by applying software and technology. He works to continually improve TEKLYNX strategic planning, product and project management principles, and cross-functional communications. When not working, he enjoys many outdoor activities with his wife and two children.
Barcode labeling is a critical aspect of business operations to get your products to the right place at the right time. When it comes to improving operational efficiency, manufacturers regularly review key performance indicators like cycle time, on-time delivery, and customer return rate.
A common labeling challenge I’ve been hearing recently is the need to move toward a paperless label approval process. Instead of printing out sample labels, physically delivering them to each person in the approval process, and returning to the label design software to implement changes, companies are looking to digitally transform this process and make label approval paperless.
READ MORE
When it comes to your barcode labeling software solution, implementing lean labeling and continuous improvement principles to your labeling environment reduces waste in label printing and saves you money.
© Copyright 2025 TEKLYNX CORPORATION SAS. Wszystkie prawa zastrzeżone.
What do you think? Leave us a comment.
Comments will be reviewed and are subject to TEKLYNX’ comment policy. Your email address will not be published publicly.