
In today's healthcare landscape, ensuring the safety of patients and maintaining an efficient supply chain is critical for medical device manufacturers. The Unique Device Identifier (UDI) system is a key component in achieving this. But what exactly is UDI labeling, why is it important, and how can manufacturers comply with its regulations?
This blog will guide you through UDI labeling regulations, breaking down the essentials for compliance. For a deeper dive into the specifics of medical device, pharmaceuticals, and healthcare labeling requirements and best practices, check out our eBook: Life Sciences Labeling & Compliance.
The UDI system final rule, established by the Food and Drug Administration (FDA), requires medical device labelers—typically, the manufacturer—to include a UDI on device labels and packages and submit device information to the Global Unique Device Identification Database (GUDID).
This helps adequately identify medical devices sold in the US from manufacturing through distribution to patient use. Medical devices range from medical gloves and tongue depressors to pacemakers and robotic surgical systems.
UDI labeling is not just about regulatory compliance—it's about safety and efficiency.
UDI is part of a global push towards harmonizing medical device regulations, with standards adopted by the FDA and the European Medical Device Regulation (EU MDR).
A UDI has two parts:
These pieces of information help track the device’s production and lifespan, making it easier to manage and recall devices when necessary.

Example of a GS1 easily readable plain-text UDI:
(01)51022222233336(11)141231(17)150707(10)A213B1(21)1234
To comply with UDI labeling regulations, manufacturers must meet specific requirements in how the UDI is displayed on their devices and packaging. Here are the key requirements:
All UDIs must be displayed on labels and packages in both human-readable (plain text) and machine-readable formats. The machine-readable portion is typically encoded using automatic identification and data capture (AIDC) technology, such as barcodes or Radio Frequency Identification (RFID).
Any date on a device label, such as the expiration or manufacturing date, must follow a standardized format: YYYY-MM-DD. This ensures consistency and clarity across the global medical device market.
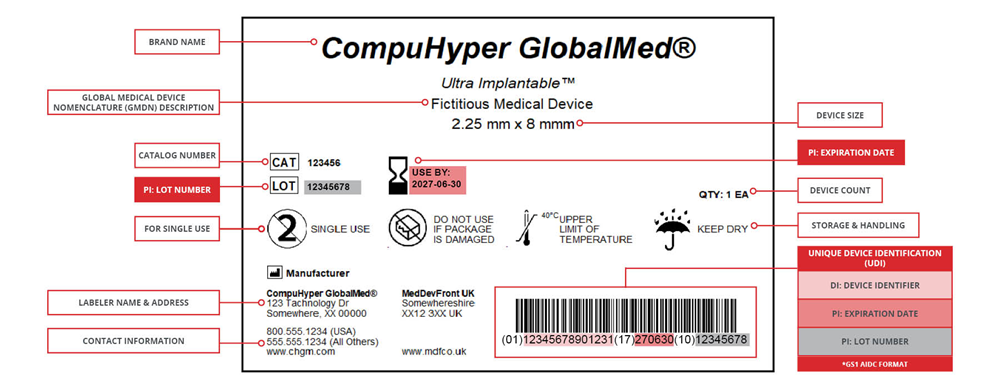
Example of a UDI-compliant label created in CODESOFT barcode label software.
Compliance with UDI regulations can seem daunting, but by following a clear process, manufacturers can ensure they meet FDA requirements.
The first step in creating UDI labels is contacting an FDA-accredited issuing agency. The FDA has approved three issuing agencies to operate a system for assigning UDIs:
Labelers must contact one of the issuing agencies directly to use their system for issuing UDIs for their devices.
After obtaining the UDI, manufacturers must submit device information to GUDID. This database is publicly accessible and serves as a reference for healthcare professionals, regulators, and patients to identify medical devices.
GUDID only includes the DI part of the UDI, which is used to look up information about the device in the database. While GUDID doesn't store PIs, it has flags to show which PI details are part of the UDI.
For more information on accessing GUDID, you can visit the FDA's website.
Make sure your labeling software can support both human- and machine-readable formats. It should also handle various barcode types, such as GS1-128 or GS1 DataMatrix, and offer the flexibility to generate unique serial numbers or batch numbers for PIs.
If your labeling software doesn't support these features and functionality, it's time to upgrade. Solutions like CODESOFT RFID and barcode label design software offer dynamic label creation based on the device's unique identifiers (DI, PI) and integrate easily with databases like Excel and Access or business systems like enterprise resource planning (ERP) systems and product lifecycle management (PLM) systems.
Download a free 30-day trial of CODESOFT and use these helpful video instructions:
During your trial, you can also access TEKLYNX Customer Support at +1 (414) 837-4800 or [email protected] if you have any questions.
Your printer must also be capable of producing high-quality labels with readable barcodes to meet UDI standards. Choose a printer brand that's compatible with your label software and offers reliable performance.
TEKLYNX has developed over 4,000 native label printer drivers for brands like Zebra, TSC, SATO, Epson, and Honeywell to provide the best overall quality for your barcode labeling needs. Learn about the benefits of using TEKLYNX printer drivers in our white paper.
By following these steps, you can ensure your medical devices meet all UDI labeling requirements, keeping your products compliant and patients safe.
UDI labeling is about making healthcare safer and more efficient. From improved product traceability to streamlined recalls, the benefits of UDI labeling extend far beyond regulatory compliance.
If you're looking for a labeling software provider to help with UDI compliance, consider TEKLYNX. Our solutions offer seamless integration with your existing systems, support for all UDI label formats, and reliable customer support. As Todd Engelken from QTS put it: "We have very high confidence in our compliance with UDI standards. I can absolutely say we made the right choice with TEKLYNX."
With the right tools, knowledge and support, complying with UDI labeling regulations doesn’t have to be difficult. TEKYNX is here to help.
Or download a free 30-day trial of CODESOFT, which includes a UDI label sample.
Jenna Wagner, Global Marketing Director, is a successful strategic marketing executive with over 20 years of marketing experience in software technology and consulting services. She is a creative, dynamic, results-driven leader who possesses a passion for developing her teams. She leverages her deep understanding of the solutions and industries she serves to deliver impactful customer value throughout the global supply chain to help organizations barcode better.
This blog will examine barcode serialization, looking at its fundamental concepts, the benefits it can offer to businesses, and its broad applications across multiple industries. Whether you are in a regulated or non-regulated industry, learn how barcode serialization can shape the future of your business.
READ MORE
If you encountered a labeling error today, would you be able to confidently pinpoint how and why it took place? Unfortunately, for many companies, the answer is “no.” Many companies have a label design and approval process that is informal, manual, and prone to mistakes.
So, how can you reduce the risk of labeling errors to near zero? You can set your company up for success by implementing an automated label audit trail. A label audit trail is a reliable, consistent way of documenting changes made to label designs. Maintaining an accurate label audit trail reduces the risk of labeling errors and helps pinpoint the cause if they happen.
READ MORE
Printing one label might sound simple, but many companies need to print thousands of labels per day. This can be a challenge when those labels must have unique data. As difficult as it might sound, printing thousands of unique labels per day can be easy with the correct enterprise labeling software.
READ MORE
What do you think? Leave us a comment.
Comments will be reviewed and are subject to TEKLYNX’ comment policy. Your email address will not be published publicly.