בחרו את האזור והשפה שלכם כדי לחוות את אתר האינטרנט באופן מיטבי

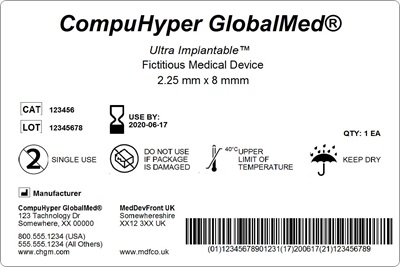
The medical device industry is one of the most highly regulated industries out there. It’s crucial when choosing the best labeling software for medical devices that your solution has the necessary features to keep your business running efficiently. Your labeling software solution should help your company comply with global medical device labeling regulations.
Here are two US Food and Drug Administration (FDA) labeling regulations the medical device industry faces that are critical for any company distributing medical devices in the US to comply with:
Becoming UDI compliant includes three components:
When you have the right labeling software for medical devices, becoming compliant and implementing UDI is easy. TEKLYNX software has built-in HIBC and GS1 barcode generator wizards that make getting (and staying) compliant easy.
Your labeling software should be able to support database connections and variable data to make managing your UDI labels simpler, more efficient, and less error prone. Your software should also include full barcode and RFID support. With robust database connection capabilities and RFID support, CODESOFT is a powerful label design application solution for UDI label design and management.
Want to learn more about UDI labeling for medical devices? Check out our eBook, Life Sciences Labeling & Compliance.
Established to protect the public health and to ensure accuracy of electronic medical records, FDA 21 CFR Part 11 applies to the research, manufacturing, and distribution of medical products. FDA 21 CFR Part 11 enables organizations to reduce costs by using electronic records in lieu of paper.
There are 7 elements to FDA 21 CFR Part 11:
In such a highly regulated environment, it’s crucial to have a barcode labeling software solution that keeps you compliant and running efficiently. TEKLYNX CENTRAL CFR is an enterprise label management solution designed specifically to help organizations comply with FDA 21 CFR Part 11. It enables users to create complex barcodes such as the 2D DataMatrix barcode, Health Industry Bar Code (HIBC), GS1 Databar, and more. To ensure your environment is secure and compliant, establish user permissions and apply electronic signatures to your labels throughout their entire lifecycle.
To learn more about TEKLYNX CENTRAL CFR, request a free demo.
The EU MDR was designed to ensure public health and patient safety across Europe and to increase quality and transparency of medical devices in the healthcare supply chain through label design, label approval, and label tracking standardization. Within the healthcare industry, many organizations who have medical devices that are implantable, life-supporting, or life-sustaining, must also bear a Unique Device Identifier (UDI) on its labels.
This includes:
UDI issuing agencies for EU MDR are:
All medical device manufacturers doing business in Europe must comply with EU MDR guidelines by May 26, 2021.
 MicroVention is a leading medical device company headquartered in California. A world-renowned developer, manufacturer and marketer of innovative neuroendovascular technologies, MicroVention operates facilities in California, Costa Rica, and China. After experiencing rapid growth and expanding distribution into international markets, MicroVention realized they needed a more efficient and controlled labeling solution.
MicroVention is a leading medical device company headquartered in California. A world-renowned developer, manufacturer and marketer of innovative neuroendovascular technologies, MicroVention operates facilities in California, Costa Rica, and China. After experiencing rapid growth and expanding distribution into international markets, MicroVention realized they needed a more efficient and controlled labeling solution.
MicroVention collaborated with the TEKLYNX Enterprise Team to determine that a browser-based, integrated enterprise label management system, TEKLYNX CENTRAL CFR, would be a scalable and efficient solution for managing its multiple facilities and providing bandwidth for future growth. After implementation took place, MicroVention streamlined its entire labeling process, resulting in a 50% improvement in production efficiency. They also saw major improvements in their ability to comply with UDI regulations as well as reduced risk of human error by using database-driven templates.
Read the MicroVention case study.
Looking for the best labeling software for your medical devices? We’re here to help!
TEKLYNX helps you find a complete labeling software solution to meet every one of your needs.
Nick Recht is the Sales Manager for the Americas region at TEKLYNX RFID and barcode label solution provider. He leverages his passion for using technology to add value to businesses and his 15 years of AIDC experience to help organizations of all sizes barcode better. When he is not working, he is driving one of his daughters to a practice of some sort or doing a project around the house.
At first glance, the custom option to almost anything sounds like the ideal solution. It's tailored to your needs exactly - what more could you ask for. If you're looking to save money and reduce unnecessary headaches, custom designed solutions might not be the right fit for you.
READ MORE
When it comes to your barcode labeling software solution, implementing lean labeling and continuous improvement principles to your labeling environment reduces waste in label printing and saves you money.
Does your company have a labeling goal of printing faster? Most companies do since faster print speeds ultimately create efficiency. To help your company begin achieving its labeling goal, TEKLYNX has developed three labeling best practices focused on streamlining the printing process to create faster print speeds. Let's start with the best practice that focuses on the core way print speeds are affected: communication.
Read More
What do you think? Leave us a comment.
Comments will be reviewed and are subject to TEKLYNX’ comment policy. Your email address will not be published publicly.