Sélectionnez votre localisation et votre langue pour la meilleure expérience sur notre site internet
Sélectionnez votre localisation et votre langue pour la meilleure expérience sur notre site internet

Life sciences companies have a lot of factors to consider when it comes to labeling, including changes in regulations on an ongoing basis from authorities like the United States (US) Food & Drug Administration (FDA) and the European Union (EU) to protect public health and improve patient safety.
EBOOK: LIFE SCIENCES LABELING & COMPLIANCE
This blog will go through global labeling regulations, compliance, labeling system validation, and labeling and artwork management solutions to support it all.
DSCSA enhances FDA’s ability to help protect consumers from exposure to drugs that may be counterfeit, stolen, contaminated, or otherwise harmful. These requirements will also improve detection and removal of potentially dangerous drugs from the drug supply chain to protect consumers.
21 CFR Part 312 Section 312.6 contains requirements for the labeling of investigational new drugs intended for human use. Information accompanying clinical investigations of that drug – or clinical trials – is critical in ensuring the safety of participants.
21 CFR Part 11 applies to the research, manufacturing, and distribution of medical products, and was established to protect public health and ensure accuracy of electronic medical records. It also enables organizations to reduce costs by using electronic records in lieu of paper.
UDI adequately identifies medical devices sold in the US market from manufacturing through distribution to patient use, which ultimately improves patient safety.
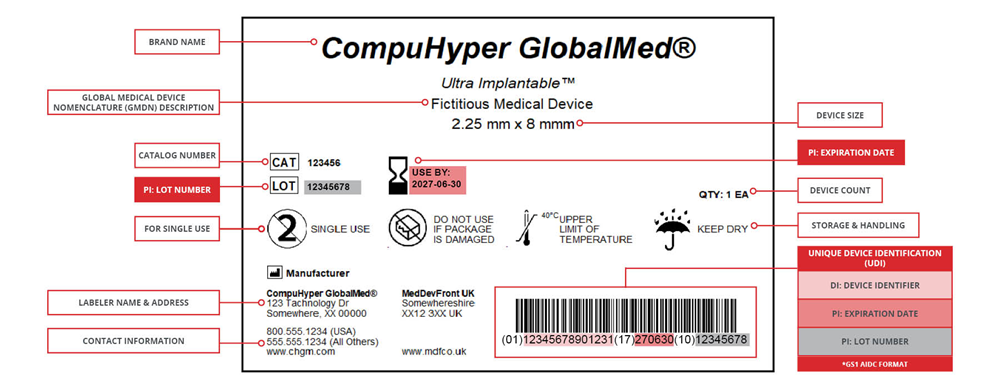 Example of a UDI-compliant label created in CODESOFT barcode label software.
Example of a UDI-compliant label created in CODESOFT barcode label software.
EU MDR is designed to ensure public health and patient safety across Europe and to increase quality and transparency of medical devices in the healthcare supply chain through label design, approval, and tracking standardization.
Compliance with life sciences labeling regulations requires proper label design, security, traceability, and version control.
Users should be able to design, manage, and print medical device, pharmaceutical, clinical trial, lab sample, and any needed labels without calling IT. This allows companies to quickly respond to evolving regulations and the development or acquisition of a new product. Speed to market is not only about quickly making changes to labels but configuring the approval process to ensure approvals are done correctly the first time and unauthorized labels don’t make it into production.
To learn more about how to manage compliance labeling, read our blog on how to manage compliance labeling.
 Validation for Quality Assurance & Auditability
Validation for Quality Assurance & Auditability
Labeling software validation is one way to ensure the tools you use when manufacturing or distributing medical and healthcare products are up to the task. In highly regulated industries, regulatory bodies like the FDA and EU have guidelines related to process validation to confirm a company’s labeling processes will result in reliable outputs and meet quality standards.
This is done through Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) protocols to establish documented evidence that the software is correctly installed, operates according to requirements, and performs safely.
“FDA software validation is when an FDA-regulated company demonstrates and documents that their software can accurately and consistently produce results that meet predetermined guidelines for compliance and quality management.” (Datacor)
The least labor-intensive tactic to life sciences labeling software validation is to work directly with your labeling system provider, who can provide a validation accelerator pack (VAP). The TEKLYNX VAP, or IQ/OQ/PQ documentation, is comprised of templates outlining the list of acceptance criteria, testing instructions, expected results, and worksheets for documentation of actual results of testing by the validation team.
An audit of your operation may be completed by the FDA for a variety of reasons, such as a routinely scheduled inspection or in response to a reported problem. It is in your, and your company’s, best interest to ensure you fully understand the FDA’s audit procedures and have the necessary documentation prepared in advance of any visits.
Top Clean Injection, the subsidiary of Top Clean Packaging, specializes in the co-design and production of medical devices through the expert knowledge of plastic injection, assembly, sealing, and packaging in clean controlled environments. As a medical device sub-contractor of approximately 250 products, Top Clean Injection must comply with specific standards and regulations, including EU MDR and UDI.
Combined, CODESOFT plus LABEL ARCHIVE supported their label traceability requirements and patient safety needs. The TEKLYNX VAP, or IQ/OQ/PQ documentation, was also key post-implementation. As a result, Top Clean Injection:
Leveraging TEKLYNX Professional Services, only two Top Clean Injection employees were required in the validation of LABEL ARCHIVE from start to finish. What would have taken approximately two weeks of complete focus to prepare the validation templates and execute validation by multiple people, only took the two Top Clean Injection staff 1.5 days. Read the full case study.
Heavily regulated industries such as medical device, pharmaceuticals, and other companies in life sciences benefit the most from leveraging the right solutions and software providers.
Cloud-enabled label and artwork management software is an end-to-end life sciences labeling solution that can accommodate the entire labeling lifecycle of highly regulated industries. It enables companies to efficiently package and label their products with required data and brand imagery across a variety of assets, patient leaflets, master data sheets, labels, artworks, promotional materials, blister packs, cartons, cases, xml content for websites, symbols, logos, barcodes, tables, die cuts, and artwork-based background images.
TEKLYNX and our strategic integration partners provide reliable and scalable solutions, plus industry-leading customer support, to help you meet virtually any life sciences labeling regulation, support business expansion, and improve patient safety. Whether your company needs software to support label and artwork management, label security and traceability, or centralized label management, plus labeling software validation for TEKLYNX solutions, we can help.
Jenna Wagner, Global Marketing Director, is a successful strategic marketing executive with over 20 years of marketing experience in software technology and consulting services. She is a creative, dynamic, results-driven leader who possesses a passion for developing her teams. She leverages her deep understanding of the solutions and industries she serves to deliver impactful customer value throughout the global supply chain to help organizations barcode better.
Compliance labeling is critical for manufacturers and distributors in many industries. Managing compliance labeling doesn’t need to cause headaches when you use a robust and reliable enterprise labeling system. Following these key principles will set you up for compliance labeling success.
READ MORE
Quality assurance (QA) is critical in manufacturing to ensure safety for employees and consumers of the product being manufactured. In highly-regulated industries like medical device manufacturing, pharmaceuticals, and life sciences, regulatory authorities like the United States Food & Drug Administration (FDA) and the European Union (EU) have guidelines related to process validation to confirm a company’s processes will result in reliable outputs and meet all necessary quality standards.
READ MORE
How three different medical device companies implemented security and traceability into their labeling process.
READ MORE© Copyright 2025 TEKLYNX CORPORATION SAS. Tous les droits sont réservés.
What do you think? Leave us a comment.
Comments will be reviewed and are subject to TEKLYNX’ comment policy. Your email address will not be published publicly.